Medication to Treat Opioid Abuse Vastly Underused; About 1 out of 5 Patients Continue to Access Opioids after Discharge from Opioid Use Disorder Inpatient Treatment
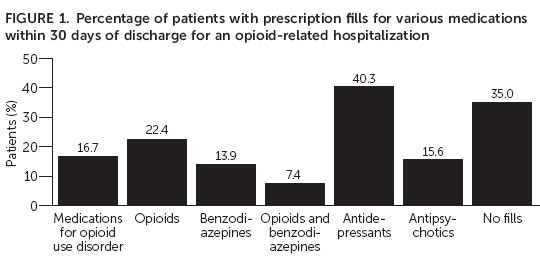
ARLINGTON, Va. – A data analyses of U.S. patients who had an opioid-related inpatient hospitalization showed that less than 17 percent received FDA-approved medication for opioid use disorder in the 30 days after discharge. In addition, 22 percent filled an opioid painkiller prescription during the same period. The research is published online today in Psychiatric Services in Advance.
Researchers, led by Sarah Naeger, Ph.D., M.P.H., with the Substance Abuse and Mental Health Services Administration (SAMHSA), studied prescription data on more than 35,000 people ages 18 to 64 who had been hospitalized for opioid abuse, dependence, or overdose between 2010 and 2014. U.S. Food and Drug Administration (FDA) has approved three medications to treat opioid dependence: methadone, naltrexone, and buprenorphine. These medications reduce illicit opioid use, reduce cravings, provide relief from opioid-withdrawal symptoms and increase treatment adherence.
The researchers looked at prescriptions filled in the 30 days following discharge for any of the three FDA-approved medications and also for four other classes of medications: antidepressants, antipsychotics, benzodiazepines (primarily used to treat anxiety), and opioid pain medications.
The researchers found that only 17 percent received a prescription for any of the three medications used to treat opioid use disorder in the 30 days after discharge. Of the other medications, antidepressants were the most commonly filled prescription (40 percent). Antipsychotic prescriptions were filled by 16 percent patients and benzodiazepines were filled by 14 percent of patients. Just over a third of patients (35 percent) did not fill any prescriptions in the 30 days following discharge.
More than one in five patients (22 percent) filled a prescription for opioid pain killers in the 30 days following hospital discharge. The authors speculate that the doctors may not have known about the patients’ hospitalization and continued prescribing the opioid painkiller. More than 7 percent of the patients in the sample filled prescriptions for both a benzodiazepine and an opioid pain medication. The combination of these two medications is not recommended because of increased risk of serious or even life-threatening problems.
These results can help inform development of targeted prevention, intervention, and treatment options for patients with opioid use disorders, the researchers note. The authors conclude that more effort is needed to ensure that patients hospitalized for opioid misuse are receiving recommended services, including approved medication and therapeutic services.
The American Psychiatric Association has joined an American Medical Association-led task force aimed at curbing the opioid epidemic. The task force has endorsed the use of state-based prescription drug monitoring programs (PDMPs) to help physicians in their decision-making process when considering treatment options.
 Note: Categories not mutually exclusive.
Note: Categories not mutually exclusive.
The American Psychiatric Association is a national medical specialty society whose more than 36,500 physician members specialize in the diagnosis, treatment, prevention, and research of mental illnesses, including substance use disorders.
Mission
To advance the quality and effectiveness of psychiatric care through advocacy, professional education and camaraderie.
Contact Us
info@ncps.org
(415) 334-2418
(415) 239-2533
77 Van Ness, Suite 101, #2022
San Francisco, CA 94102
